Exploring Innovative Bond Transformations in Nitrogen Activation and Boron Chemistry
In an impressive display of chemical innovation, a team led by Felipe Fantuzzi (SISC) and Holger Braunschweig (Julius-Maximilians-Universität Würzburg) has published a groundbreaking study in the Journal of the American Chemical Society titled Boron Insertion into the N≡N Bond of a Tungsten Dinitrogen Complex. This research marks a significant stride in understanding and manipulating the chemistry of nitrogen and boron, elements crucial to many areas of science and technology.
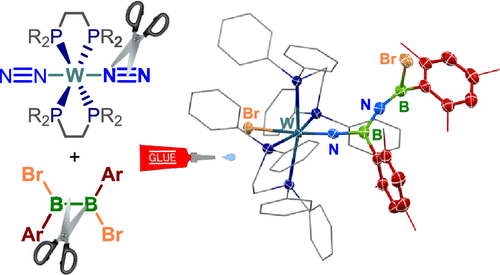
The study revolves around the 1,3-addition of 1,2-diaryl-1,2-dibromodiboranes (B2Br2Ar2) to trans-[W(N2)2(dppe)2] (dppe = κ2-(Ph2PCH2)2). This reaction, fascinatingly accompanied by a Br–Ar substituent exchange between the two boron atoms, is followed by a spontaneous and complex rearrangement. The tungsten diboranyldiazenido complex thus formed undergoes a transformation into a 2-aza-1,3-diboraallenylimido complex, showcasing a linear, cumulenic B=N=B moiety.
This remarkable rearrangement involves the cleavage of both B–B and N=N bonds of the N2B2 ligand, formal insertion of a BAr boranediyl moiety into the N=N bond, and coordination of the remaining BArBr boryl moiety to the terminal nitrogen atom. Through sophisticated density functional theory calculations, the team has elucidated that the reaction proceeds via a cyclic NB2 intermediate. This then dissociates into a tungsten nitrido complex and a linear boryliminoborane, which subsequently recombine, forming a unique adduct.
Moreover, the linear B=N=B moiety undergoes facile 1,2-addition of Brønsted acids (HY = HOPh, HSPh, and H2NPh), leading to a Y–Br substituent exchange at the terminal boron atom and yielding cationic (borylamino)borylimido tungsten complexes.
This study does not just add a new chapter to the chemistry of boron and nitrogen; it opens a whole new section. The implications of these findings are vast, ranging from potential applications in catalysis and materials science to enhancing our understanding of bond-making and breaking processes. The collaborative effort of this research team has set a new benchmark for studies in nitrogen activation and boron chemistry, promising exciting developments for future investigations.
For more information please read the research article by Felipe Fantuzzi, Holger Braunschweig, and co-authors, which can be found here.