Introducing a Novel Mechanochemical Approach for Enhancing Iron(II) Spin Crossover Materials
Researchers Jed Askew and Helena Shepherd from the SISC Group at the University of Kent have developed an innovative and facile method for post-synthetic exchange of anions in iron(II) spin crossover materials using mechanochemistry. This groundbreaking study, titled Post-synthetic anion exchange in iron(ii) 1,2,4-triazole based spin crossover materials via mechanochemistry and published in Dalton Transactions, represents a significant leap forward in the field of material science.
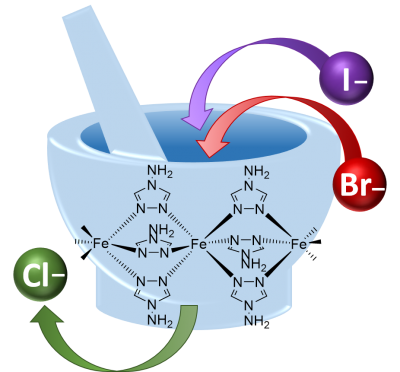
The team successfully demonstrated that dry grinding the [Fe(atrz)3]Cl2 complex (atrz = 4-amino-1,2,4-triazole) in the presence of an excess of sodium halide salt leads to the complete exchange of anions and the formation of [Fe(atrz)3]Br2 and [Fe(atrz)3]I2. This solid-state metathesis reaction offers a novel strategy for fine-tuning active switching properties, such as the transition temperature in spin crossover systems, which are crucial for various applications in sensing, memory devices, and displays.
A key insight from their research is the identification of stable by-products as a major driving force for the exchange. The study also presents a straightforward method to predict the likely outcome of such reactions using simple thermodynamic considerations, marking a significant advance in our ability to manipulate and control the properties of spin crossover materials.
This research not only opens up new avenues for the post-synthetic modification of coordination compounds but also sets a new standard for the development of responsive materials. The simplicity and effectiveness of the mechanochemical approach hold great promise for future material design and applications, ushering in a new era of material science innovation.
For more information please read the research article by Jed Askew and Helena Shepherd, which can be found here.