Research on hybrid perovskites within the Materials for Energy and Electronics (MEE) group by a team lead by Paul Saines has been featured on the cover of the European Journal of Inogranic Chemistry and recently highlighted as an ISIS Neutron and Muon Source science highlight.
Materials with the perovskite structure have a wide range of fascinating and industrially important properties including magnetism, catalysis and ionic conduction. The basic perovskite structure takes the form ABX3, incorporating larger A-site site cations within a framework made from smaller octahedral B-site cations linked through X-site anions to six B-site neighbours. The range of applications of the materials come from the fact that the structure is able to accommodate many different A, B and X ions with a range of charges.
While purely inorganic perovskites have been a focus of much study, including within the MEE group, for a number of years it is also possible to introduce organic building blocks into this structure. These inorganic-organic perovskites are often refered to as hybrid perovskites due to their ability to include organic cations on both their A- and X-sites. Examples of this include HCO2– on the X site, in a family of phases studied for their ferroelectrics and multiferroics properties, and compounds based on organic cations, such as [(CH3)2NH2]PbI3, which are promising materials for next-generation solar cells and are currently studied by other researchers within the MEE group to stabilise their functional cubic states by iodine treatment.
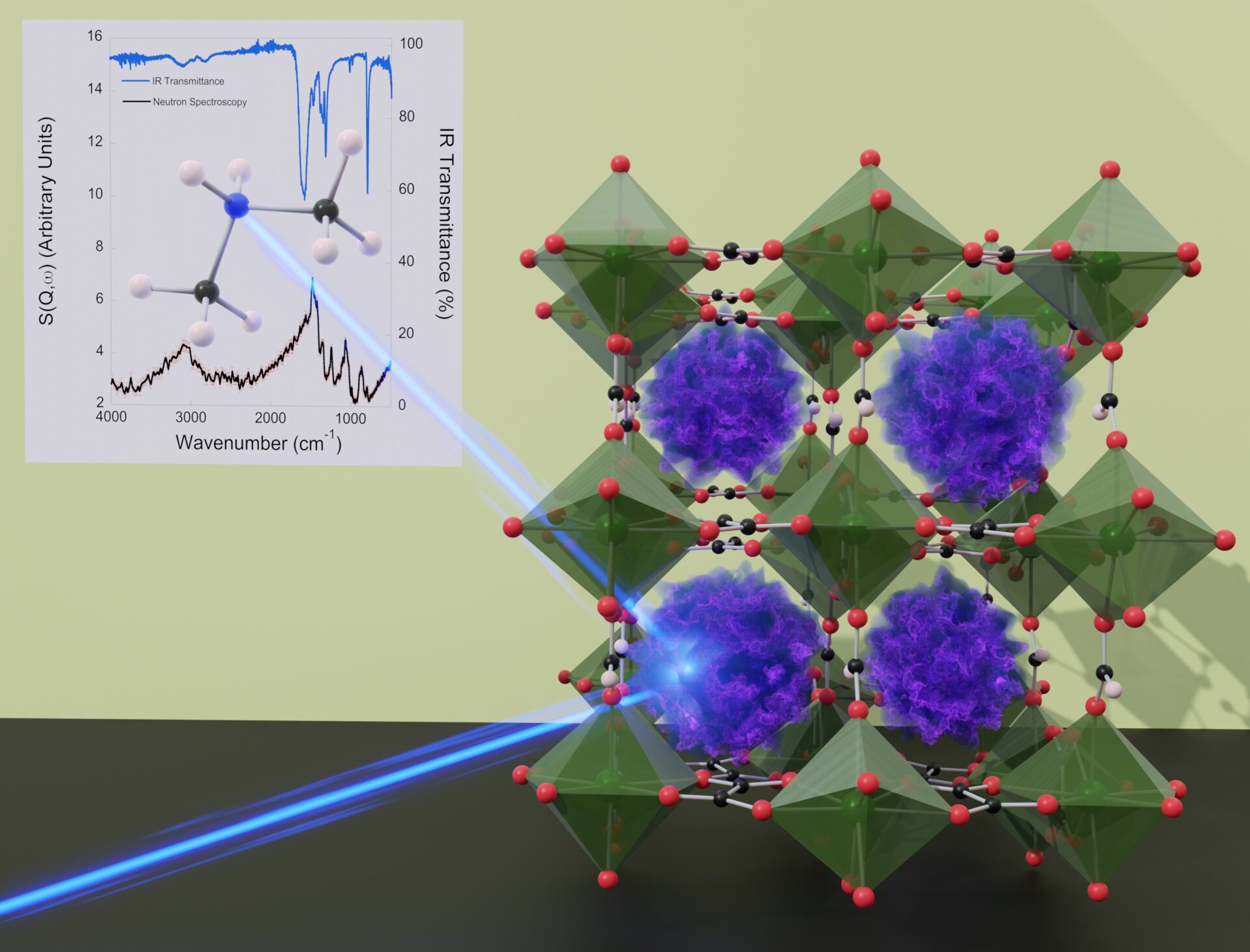
Research that formed part of the Masters thesis of Lydia Burley who was supervised by Paul Saines made hybrid perovskites with the composition ALn(HCO2)(C2O4)1.5 (where Ln represents a lanthanide, in this case Tb, Dy, Ho or Er). This family of materials are the first time a hybrid perovskite has featured ordered anion vacancies combined with an A-site cation with a single charge and a triply charged B3+ ion. Unfortunately the A-site cation was extensively disordered, a not uncommon problem in related materials, such that the A-site cation could not be identified from the crystal structure of the material. A solution of this was found by using inelastic neutron scattering (INS) on the Tosca Instrument at ISIS, in collaboration with Svemir Rudić, which allowed the A site cation to be clearly identified as [(CH3)2NH2)]+, with support from infrared spectroscopy.
This study shows the importance of INS to this area of research due to the techniques high sensitivity to the bonds between hydgrogen and other atoms, which are a key part of the organic components of these materials. INS offers the potential use in a much wider range of perovskite with organic components to confirm their composition, which is important to optimise hybrid perovskites with disordered A-site cations for applications. The key role of INS in this study, which is published here featured and will be highlighted on the cover of the European Journal of Inorganic Chemistry in October, lead to the work being selected as an ISIS Neutron and Muon Source Science Highlight.