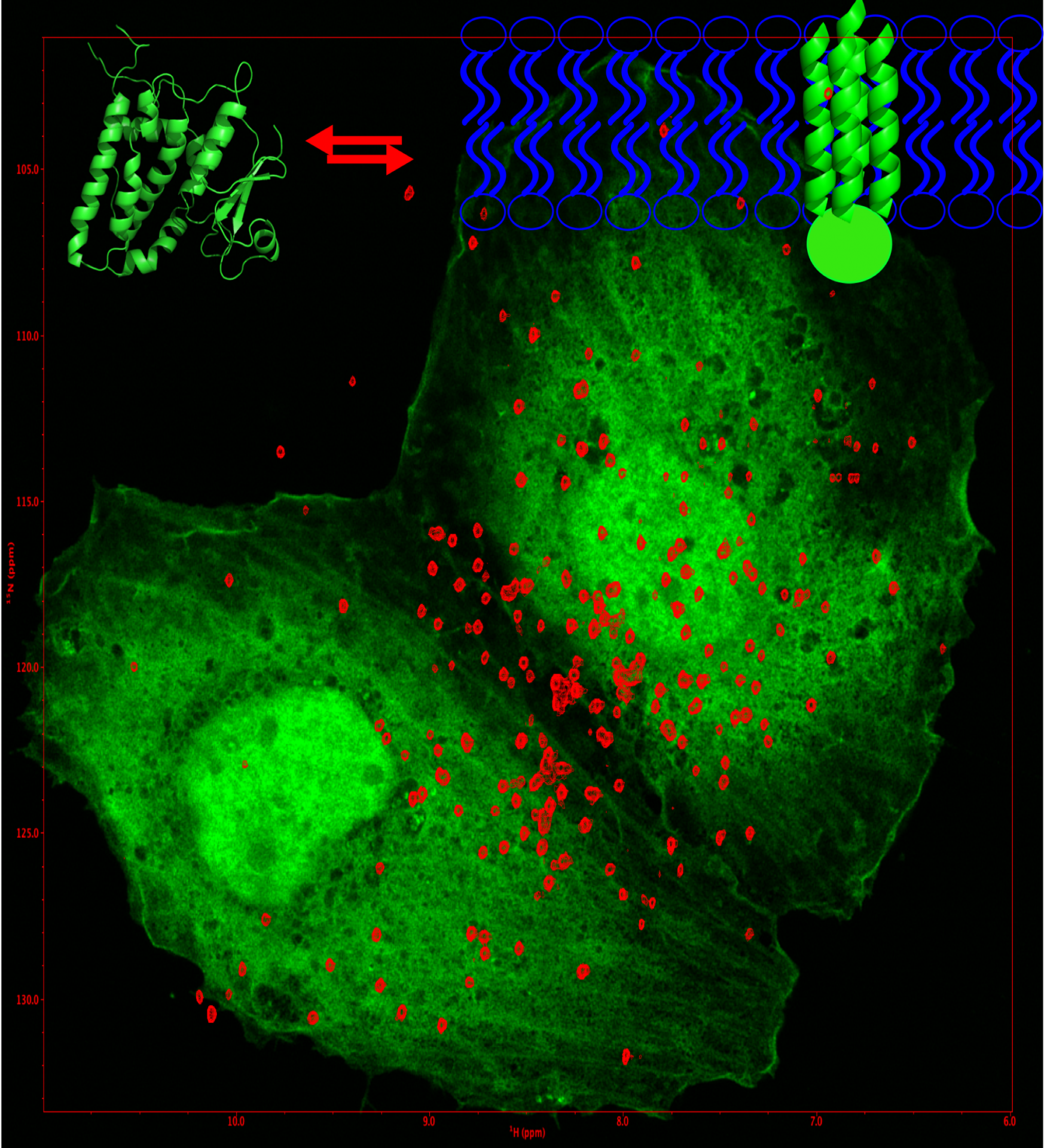
In-cell structural biology: CLIC1 structure, function and drug binding inside tumour cells
The Chloride Intracellular Channel (CLICs) proteins consist of a family of metamorphic proteins that exist in an equilibrium between a soluble and a membrane-bound state. The alteration of CLIC function has been involved in ischemia-reperfusion and different forms of cancer. CLIC1 has been directly linked with glioblastoma proliferative capacity.
The aim of my research is to study the membrane-associated and free conformations of CLIC1 by solution NMR both in-vitro and in-vivo using in-cell NMR in different cell lines in combination with traditional NMR methods and fluorescence microscopy. I seek to identify which CLIC1 conformer is involved in disease and drug binding, to understand the factors governing the equilibrium between the different CLIC1 forms and to determine the mechanism regulating such equilibrium with atomic detail. These findings will permit further studies to develop more specific therapeutic drugs against glioblastoma and other types of tumour where CLIC1 is activated. We have also adopted an integrative biology approach to elucidate the membrane-bound structure and understand inhibition of CLIC proteins.
Investigating the lipid modulation of membrane proteins activity
Membrane proteins are crucial to many cellular processes; from the import and export of nutrients and toxins, to signal transduction, environmental adaptation and energy production. Our mechanistic understanding of membrane proteins, particularly transporters and channels, has benefitted immensely from decades of biochemical studies and the relatively recent explosion of high resolution structures. However, a major blind spot in much of this work is the influence the lipid environment has on protein structure and function, the importance of which cannot be overstated; these are proteins that have evolved to be embedded in the lipid bilayer. Probing specific protein lipid interactions is made difficult by the complexity of the lipid bilayer. Variations in lipid headgroup, acyl chain length and saturation level can result in >10,000 unique lipids. We are developing new methodologies to investigate the lipid dependence of membrane proteins in their native environment.
Investigating the rupture the plasma membrane in necrosis?
The ultimate aim of this work is to establish the mechanism of cell death triggered by necroptosis with atomic detail. Necroptosis constitutes a mechanism of programmed immunogenic cell death, which is involved in the defence against pathogens. It is characterised by cellular swelling, rupture of the plasma membrane and release of cellular components into the extracellular space, resulting in inflammation and immune system activation.
Previous studies show that the protein Mixed Lineage Kinase Domain-Like Pseudokinase (MLKL) is a critical effector of necroptosis. Upon phosphorylation by the threonine protein kinase 3 (RIPK3), MLKL oligomerises, inserts into and permeabilises the plasma and organelle membranes. The structure of the cytoplasmic protein form is known, as well as mutations that modify its activity. In contrast, the mechanism of plasma membrane insertion and rupture remain enigmatic. Understanding the structure of MLKL bound to the membrane and its mechanism of insertion is crucial to understand necroptosis-mediated cell death and its links to pathogenic infection. We use an integrative structural biology approach to answer the following questions: What changes in the structure and dynamics are required for MLKL activation? How does the structure of MLKL change upon insertion into the membrane? What is the overall shape of the pore formed by MLKL?
Investigating the pharmacology of Sigma-1 receptors.
The σ1 receptor represents one of the most pharmacologically diverse and poorly understood drug targets in humans. This small (25 kDa) single transmembrane receptor is found in high abundance in the Central Nervous System (CNS) and appears to have strong neuroprotective effects and associations with a number of pathologies, ranging from stroke and cancer through to depression and addiction.
The 3D structure of this protein is known, but the changes between agonist- and antagonist-bound structures are small, leaving the structural demarcation of this classification unclear. We use a range of structural biology tools, including NMR, X-Ray crystallography and Electron Microscopy to investigate the changes is structure and dynamics that result upon agonist and antagonist binding.