Basal Cell Carcinoma (BCC), the most common type of skin cancer, presents a significant challenge to those who suffer from it. However, despite the overwhelming burden placed on dermatology outpatient services, we can continue to push for progress. With the help of reliable tools to aid in diagnosis, we can reduce unnecessary referrals and provide better care to those in need. Though the gold standard for BCC diagnosis involves biopsy, we must strive towards more efficient and effective methods to overcome this challenge together.
The handheld device depicted in the figure above is equipped with a liquid lens that allows for shifting focus inside the sample, resulting in high-resolution lateral images throughout the imaging range (the Gabor technique). Our instrument can produce images with longitudinal resolutions of approximately 5 μm, limited by the light source employed, whose spectrum spans over 45 nm at a central wavelength of 840 nm, and better than 2 μm transversely. It should be noted that the data is acquired, processed, and displayed in real time. Furthermore, the camera used in the spectrometer can operate at 70 kHz. This technological development is a significant step forward in the field of imaging. The ability to produce high-resolution images with such precision and speed is a testament to the beauty of the combination of Master-Slave OCT with the dynamic movement of the focusing position inside the sample. This combination can transform how we approach imaging and provides future research and development opportunities.

This project aims to revolutionise the diagnosis process by employing a non-conventional method that does not require tissue removal. Instead, it uses an Optical Coherence Tomography (OCT) instrument. While current OCTs cannot produce optical biopsies equivalent to conventional ones, this project will develop a portable OCT instrument that can produce high-definition images comparable to those obtained using traditional biopsies. Additionally, the instrument will capture information on the distribution of blood vessels and tissue stiffness, making this project a significant step forward in the field of diagnosis. Lastly, creating a database of BCC signatures will allow for BCC diagnosis in a clinical setting, making it an invaluable resource in the medical community.
At this project stage, our instrument has undergone extensive development and testing and is now finally ready to be put to the ultimate test: ex-vivo testing on a sample in the laboratory. This step is crucial in determining the effectiveness and reliability of the instrument, as it will simulate the real-life conditions it will face in a hospital setting. If the test results are satisfactory, we will move forward and deploy the instrument in a hospital setting, where it will have the potential to revolutionise the way medical procedures are carried out, hopefully.
Below, we present a selection of images captured by our state-of-the-art instrument. These en-face images have been obtained from various samples, including tissue paper lenses, infrared cards, and orange fruits, as well as biological samples at depths ranging from 200 to 500 μm beneath the surface. Our technology enables us to capture high-quality images that provide valuable insights into the structure and composition of these samples.

Below, you will find multiple “hyper-spectral” en-face images, colour-coded to differentiate between images from different depths. Each image has a longitudinal width of approximately 5 μm, and the number of images per sample varies depending on the object being imaged. A video showcasing a fly-through of these samples is also available at the bottom of the page.

Selection of 8 en-face images collected from within inside an IR-card
(separation between images, ~20 μm, size of the images 200×200 µm).
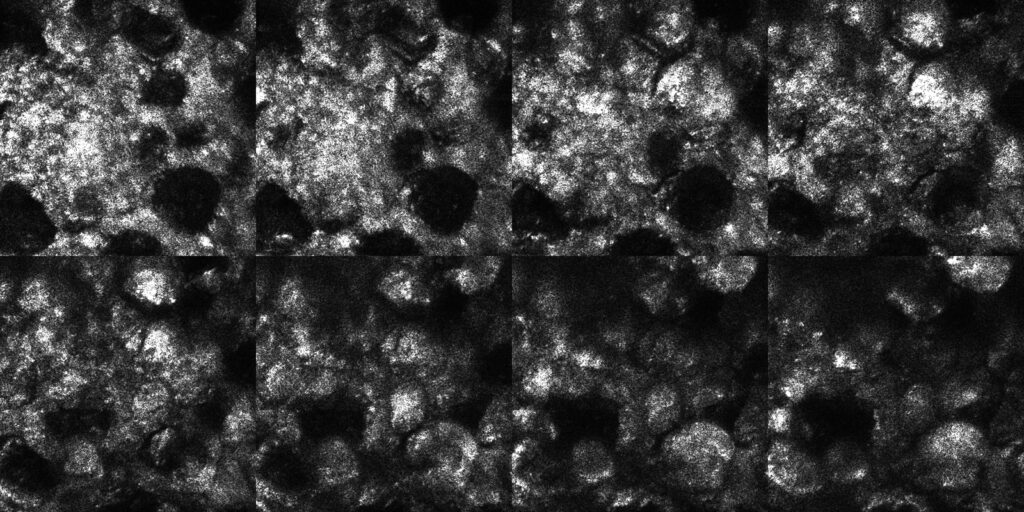
Selection of 16 en-face images collected from within inside chicken skin tissue
(separation between images, ~20 μm, size of the images, 300×300 µm).

Selection of 12 en-face images collected from within inside an orange fruit
(separation between images, ~20 μm, size of the images, 300×300 µm).

Selection of 16 en-face images collected from within inside chicken tissue
(separation between images, ~20 μm, size of the images, 300×300 µm).

